FDA Guidance on Conduct of Clinical Trials of Medical Products during COVID-19 Pandemic: Guidance for Industry, Investigators, a

Early phase clinical trials extension to guidelines for the content of statistical analysis plans | The BMJ
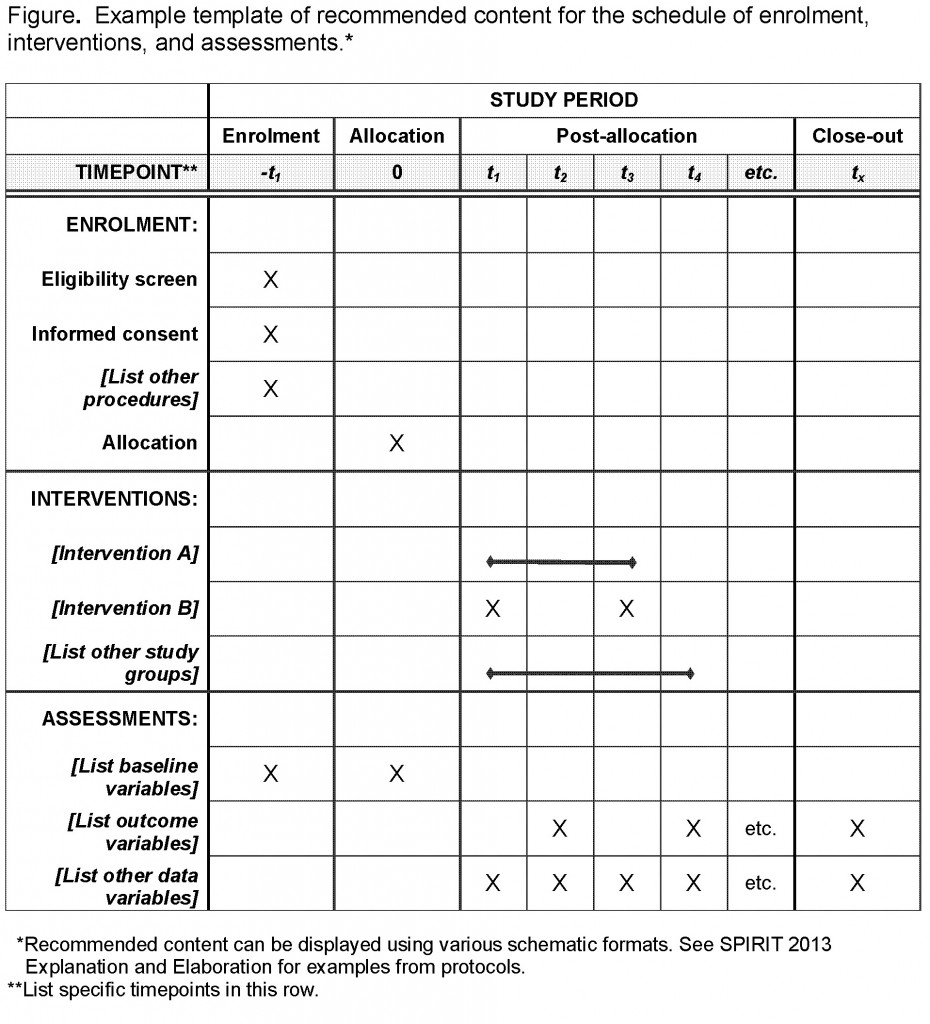
![PDF] SPIRIT 2013 statement: defining standard protocol items for clinical trials. | Semantic Scholar PDF] SPIRIT 2013 statement: defining standard protocol items for clinical trials. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/ccf1230c8d03a35229dc9fd36776d69c3356cfa3/2-Table1-1.png)
PDF] SPIRIT 2013 statement: defining standard protocol items for clinical trials. | Semantic Scholar

Guidelines for clinical trial protocols for interventions involving artificial intelligence: the SPIRIT-AI extension - The Lancet Digital Health

Guidelines for clinical trial protocols for interventions involving artificial intelligence: the SPIRIT-AI extension - The Lancet Digital Health

PPT – Clinical trial protocol writing: Challenges and Guidelines PowerPoint presentation | free to download - id: 906c6c-ODNhN

Recruitment of Black Adults into Cardiovascular Disease Trials | Journal of the American Heart Association

Guidelines for clinical trial protocols for interventions involving artificial intelligence: the SPIRIT-AI extension - The Lancet Digital Health

Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: the CONSORT-AI extension - The Lancet Digital Health