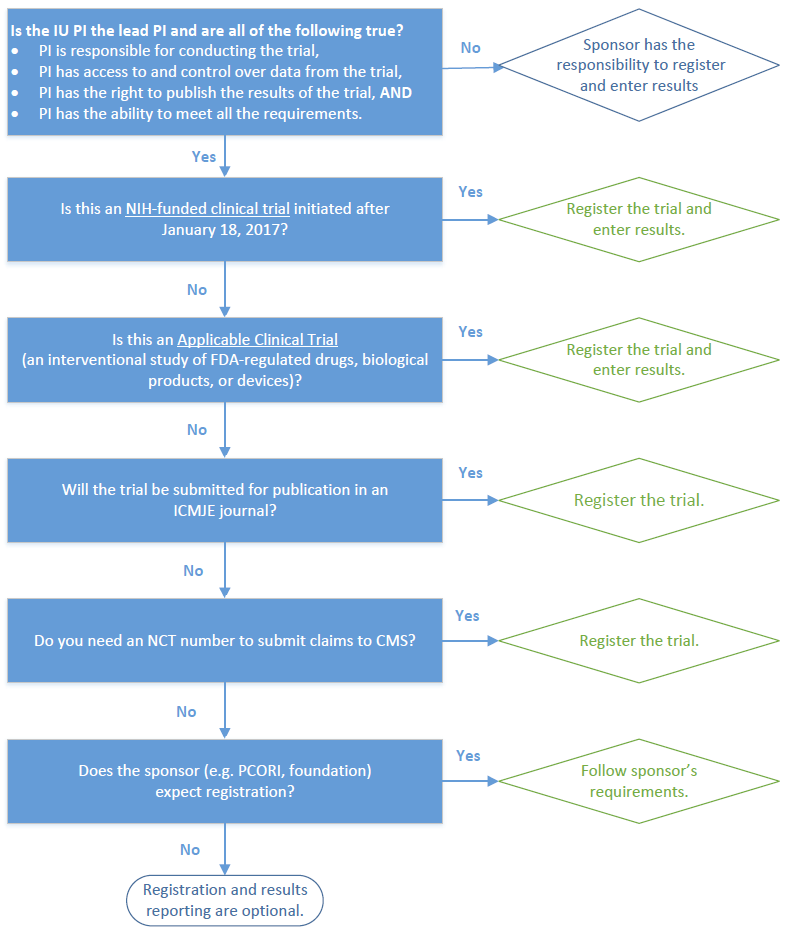
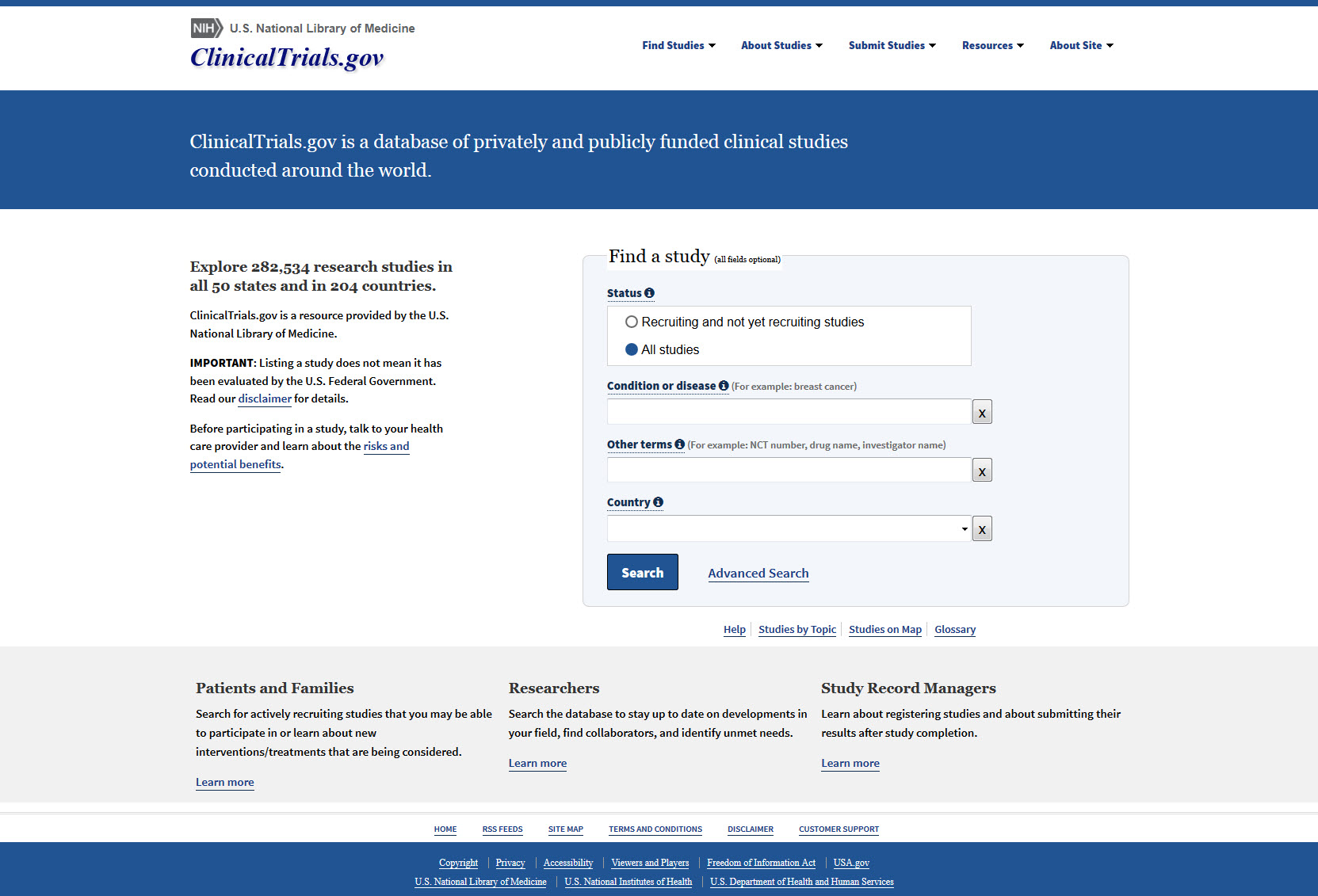
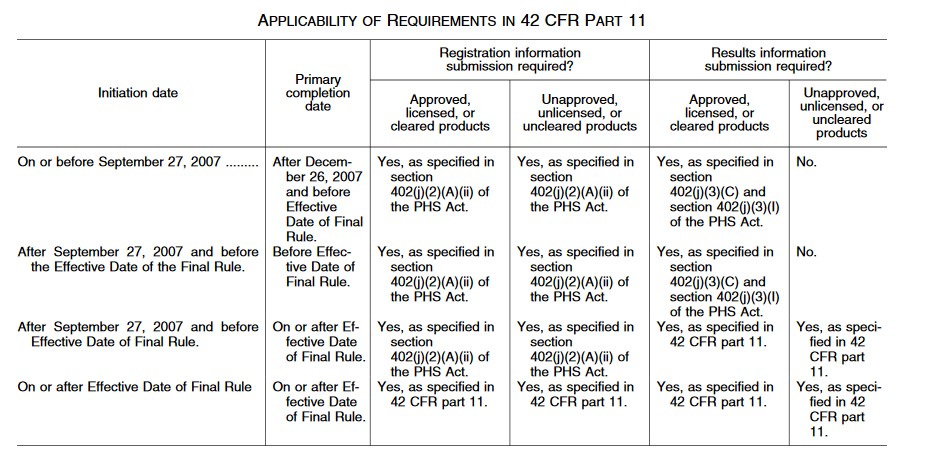
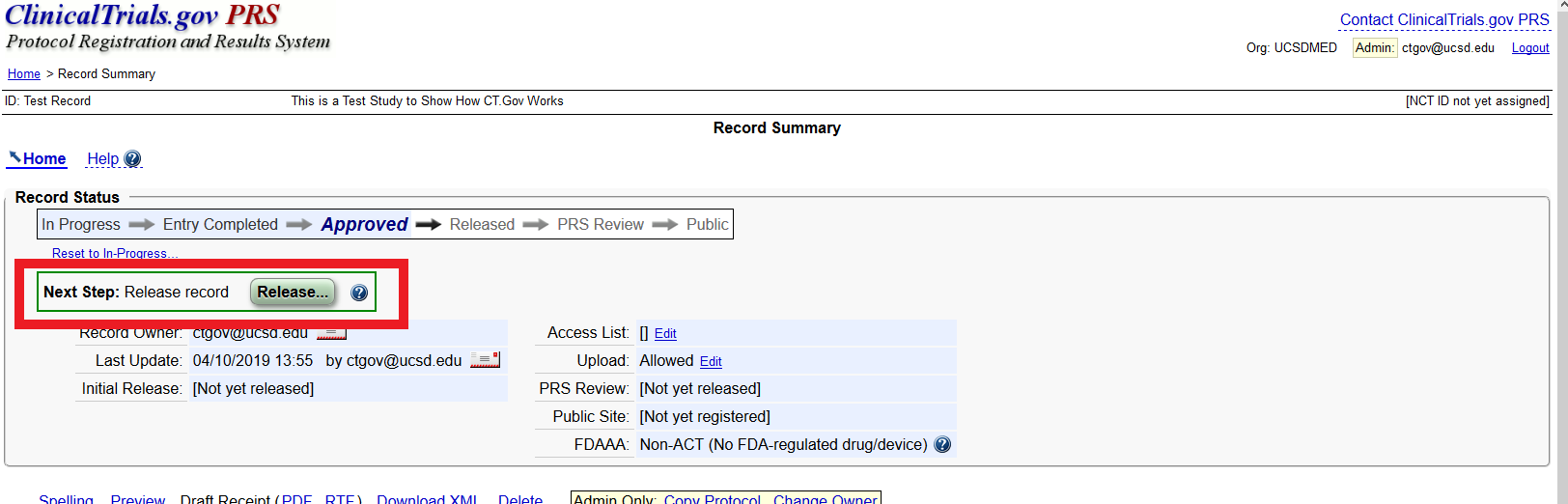
Navigating the Expanding Regulations of ClinicalTrials.gov Registration and Results Reporting - Advarra

ClinicalTrials.gov: Does Your Study Need to be Registered? - Clinical and Translational Science Institute - University at Buffalo
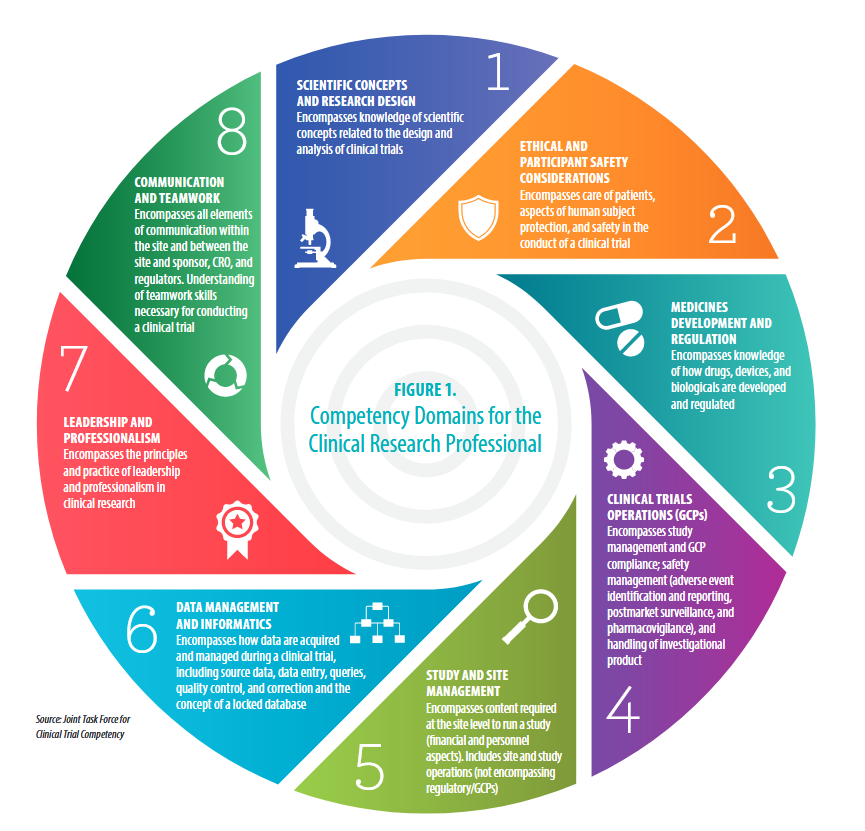
ClinicalTrials.gov Registration" | Center for Clinical and Translational Science | University of Illinois Chicago