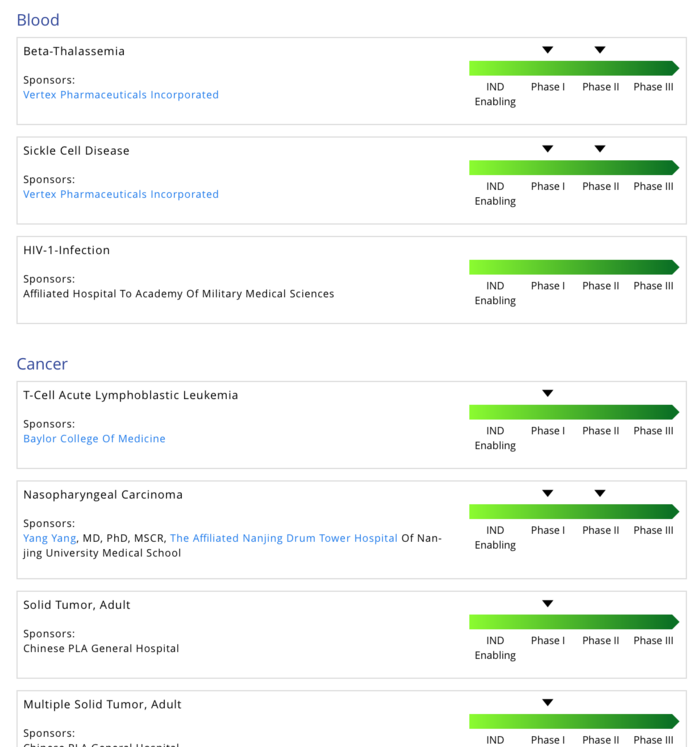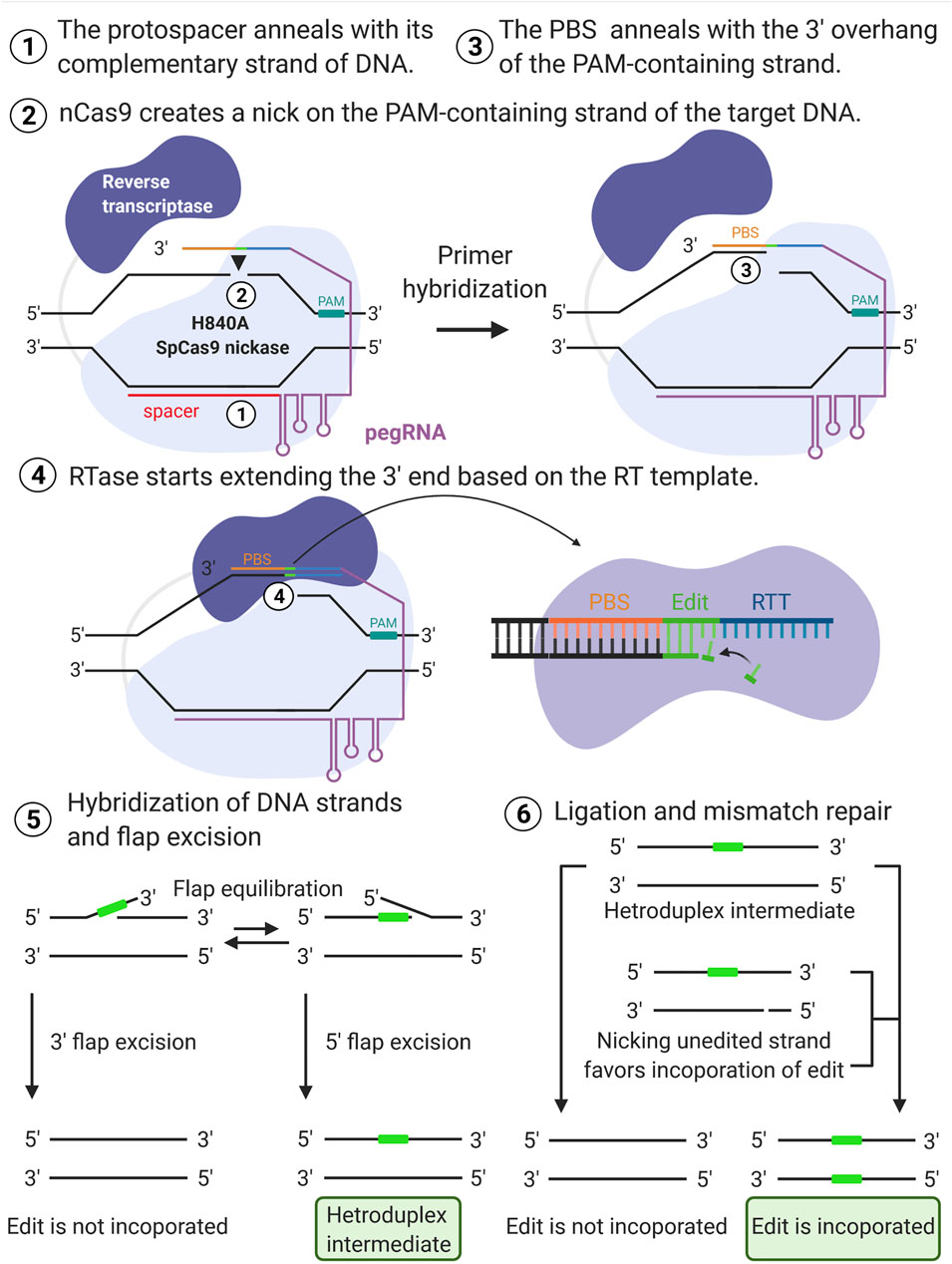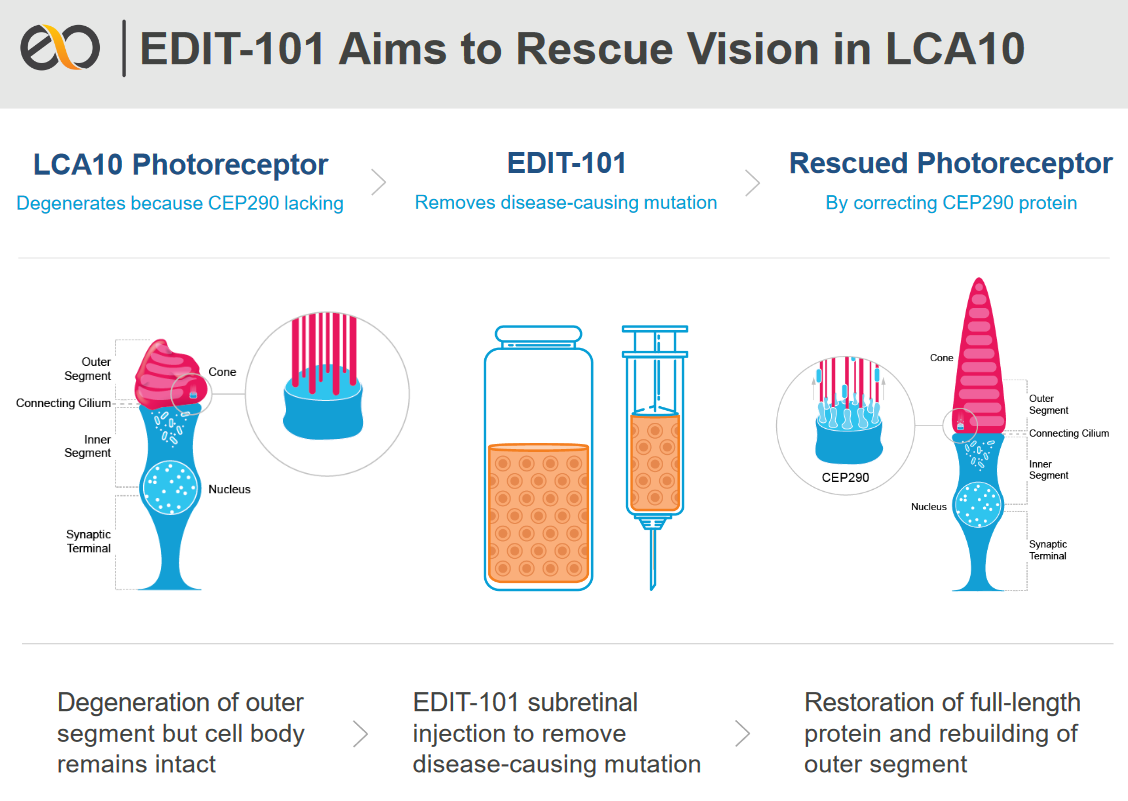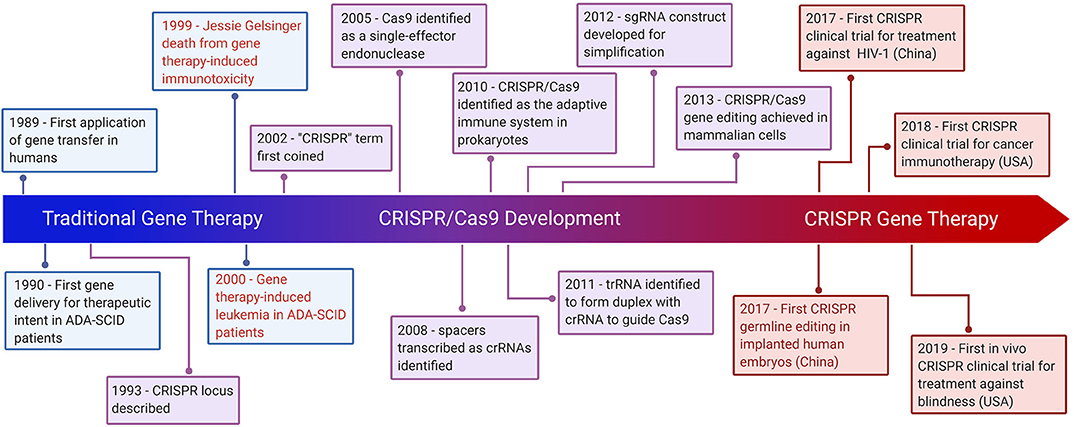
Frontiers | CRISPR Gene Therapy: Applications, Limitations, and Implications for the Future | Oncology

Delivery of CRISPR-Cas tools for in vivo genome editing therapy: Trends and challenges - ScienceDirect

Leber congenital amaurosis/early-onset severe retinal dystrophy: current management and clinical trials | British Journal of Ophthalmology

Editas Medicine Announces Dosing of First Pediatric Patient in the BRILLIANCE Clinical Trial of EDIT-101 for LCA10 | Editas Medicine

Development of a gene-editing approach to restore vision loss in Leber congenital amaurosis type 10 | Nature Medicine

Advancements and Obstacles of CRISPR-Cas9 Technology in Translational Research: Molecular Therapy - Methods & Clinical Development

The power and the promise of CRISPR/Cas9 genome editing for clinical application with gene therapy - ScienceDirect
Clinical Data from Editas Medicine's Ongoing Phase 1/2 BRILLIANCE Clinical Trial of EDIT-101 for LCA10 to be Presented at the
JPM 2022: Editas, which caught flak in 2021 for limited gene editing data, will try to layer on the proof in 2022 | Fierce Biotech

Editas and Allergan Make Gene-Editing History With First Treatment of Blindness Drug | The Motley Fool